



Next: Experimental Data
Up: Chemical Reaction Kinetics
Previous: Chemical Reaction Kinetics
Validation of Reaction Mechanisms
Currently, no known published mechanism is capable of accurately
modeling mixtures containing all of the chemical species of
interest to this study. Computational requirements and
limitations cause most mechanisms to be designed for a
particular application, and they are of uncertain value under
off-design conditions. To evaluate the usefulness of the
collected mechanisms under a variety of conditions, simulations
using the mechanisms have been compared to experimental data
from the literature. Data suitable for comparison with chemical
kinetics computational results include shock tube induction
times, flame induction distances, stirred reactor induction
times, and flame species concentration profiles. To simplify
the analysis, and because a large portion of the available data
is in the form of shock tube induction time, we concentrated on
comparisons with induction time measurements. For the sake of
numerical analysis, the chemical reactions behind both incident
and reflected shocks are
modeled as constant-volume processes. This is a good approximation in
most cases since the shocked mixtures are typically highly diluted with Argon and
there is relatively weak coupling between the chemical reactions and the
fluid motion.
In general, induction times are straightfoward to measure and there
are abundant data in the published literature.
However, there are a number of difficulties that
we encountered:
- 1.
- Induction time can be defined in a number of different ways
for the purposes of both experimental measurement and numerical
modeling (see Section 3.1.1).
- 2.
- Data from different reactant concentrations are sometimes
presented together without individual identification, making proper
modeling difficult.
- 3.
- Most validated reaction mechanisms are most accurate at lower pressures
and higher temperatures than those encountered in detonations (within the
induction zone).
- 4.
- Each validation data set or each set of reaction rate parameters
is useful for limited ranges of temperature, pressure, and species
concentrations.
- 5.
- Many investigators plot induction time data in such a way as to
remove the pressure dependence (i.e.
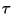 [X]), and then plot data for a
variety of pressures together. This makes it difficult to precisely
compare experimental and computational results.
[X]), and then plot data for a
variety of pressures together. This makes it difficult to precisely
compare experimental and computational results.
In Sections 3.1.3 to 3.1.9 below, a brief review
of the validation effort is given for each simple fuel-oxidizer mixture.
In some instances, different dilutions are
examined separately. Each section consists of a list of references
containing reaction mechanism or induction time data, discussion of these
references, a description of the results of the validation study,
and recommendations of appropriate conditions for use of the studied
mechanisms. These recommendations are summarized in Table 11
and supporting figures are provided in Appendix ![[*]](/l2htmlicons/gif/cross_ref_motif.gif) .
These reviews are not exhaustive.
.
These reviews are not exhaustive.
The thermodynamic condition within a fuel-air detonation typically varies
from 1500 K and 40 atm (von Neumann state) to 3000 K and 20 atm
(Chapman-Jouguet state). Since the conditions within the induction
zone are approximately the von Neumann condition and primarily
determine the length of
the reaction zone, accurate modeling of this condition is most important.




Next: Experimental Data
Up: Chemical Reaction Kinetics
Previous: Chemical Reaction Kinetics
Joe E. Shepherd
2000-01-17
![]() .
These reviews are not exhaustive.
.
These reviews are not exhaustive.