Table 3:
Induction time definitions used in the
literature
| [Asaba
et al. (1963)] |
Sudden end wall pressure rise. |
| [Bhaskaran
et al. (1973)] |
Large increase in optical emission (photomultiplier)
and end wall pressure. |
| [Blumenthal et al. (1996)] |
Multiple induction time definitions were
used, and two events were sometimes observed: an initial small change
(``first kernel'') followed by a more rapid change (DDT). The ``first
kernel'' was variably measured by detection of a density gradient
(shadowgraph), sudden OH emission, or a slight pressure rise. A
sudden pressure jump defined the DDT. In cases where no detonation occurred,
a first kernel was reported by the shadowgraph technique. |
| [Borisov et al. (1977)] and [Borisov
et al. (1978)] |
In
``static experiments'', induction time
was defined as the delay between stopping the flow and the observation of a sharp pressure
increase. In shock tube experiments, absorption spectroscopy of N2O
at 253.6 nm was used to detect a sudden decrease in N2O concentration. |
| [Bradley
et al. (1968)] |
Appearance of OH by absorption at 306.7 nm and
NH3 emission at 3000 nm. |
| [Burcat
et al. (1971)] |
Sudden level or slope change in pressure and
heat flux. |
| [Cheng and
Oppenheim (1984)] |
Extrapolated from reflected wave trajectories
(pressure increase). |
| [Craig (1966)] |
Sudden pressure rise. |
| [Drummond (1969)] |
Maximum OH absorption at
307 nm and end wall pressure rise. |
| [Drummond (1972b)] |
Absorption by OH at 307 nm, NH2 at 570 nm,
and NH at 336 nm. |
| [Hidaka
et al. (1985b)] |
tm was defined by the point of maximum OH
emission intensity at 305.5 nm. |
| [Hidaka
et al. (1985a)] |
t20, t50, and t80 are times
to 20, 50, and 80 percent consumption of N2O measured by infrared
emission at 4.68 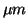 . .
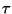 indicates a rapid decrease in N2O
concentration.
indicates a rapid decrease in N2O
concentration. |
| [Miyama and
Endoh (1967a)] |
Appearance of nitric oxide emission at
430.5 nm. |
| [Miyama and
Endoh (1967b)] |
Variation of NH3 absorption at 224.5 nm. |
| [Miyama (1968b)] |
Variation of NH3 absorption at 224.5, 230,
and 240 nm. |
| [Miyama (1968a)] |
Variation in nitric oxide emission at 430.5 nm. |
| [Pamidimukkala and
Skinner (1982)] |
Induction time was defined at maximum
O concentration by atomic resonance absorption spectroscopy. Another time
was reported as the point where the reaction was 65% complete, by an
unknown method. |
| [Petersen et al. (1996)] |
Time of maximum rate of OH formation as
indicated by absorption spectroscopy. |
| [Seery and
Bowman (1970)] |
Most rapid increase in pressure, OH absorption,
and emission of OH (306.7 nm), CH (431.5 nm), CO (220.0 nm) and C2 (516.5 nm). |
| [Skinner and
Ringrose (1966)] |
Time of maximum OH emission. |
| [Soloukhin (1971)] |
Interferograms, emission of N2O at
4.5 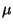 ,
and of OH at around 300 nm were interpreted to derive induction
times. ,
and of OH at around 300 nm were interpreted to derive induction
times. |
| [Takeyama and
Miyama (1967)] |
Variation in NH3 absorption at 224.5 nm. |
| [Takeyama and
Miyama (1965)] |
Variation in OH emission. |