The experimental apparatus used was the GALCIT Detonation Tube
(Figs. 1, 2, and 3), first
described in a previous report [Akbar and
Shepherd (1996)]. The tube is
constructed of three cast stainless steel (304) sections joined together
by flanges and high-strength fasteners. The assembly is 7.3-m long
and has a 280-mm inside diameter. A vacuum system is used to evacuate
the tube to less than 50 mTorr before each test.
A gas handling system can supply
H2, N2O, N2, NH3, CH4, O2, Ar, and He from a cylinder
farm located
outside the building. Gas composition is controlled by the method of
partial pressures using an electronic Heise 901a gauge, accurate to
![]() 0.18 kPa. Before a test, the test mixture is circulated through
the tube volume with a bellows pump to ensure homogeneity.
0.18 kPa. Before a test, the test mixture is circulated through
the tube volume with a bellows pump to ensure homogeneity.
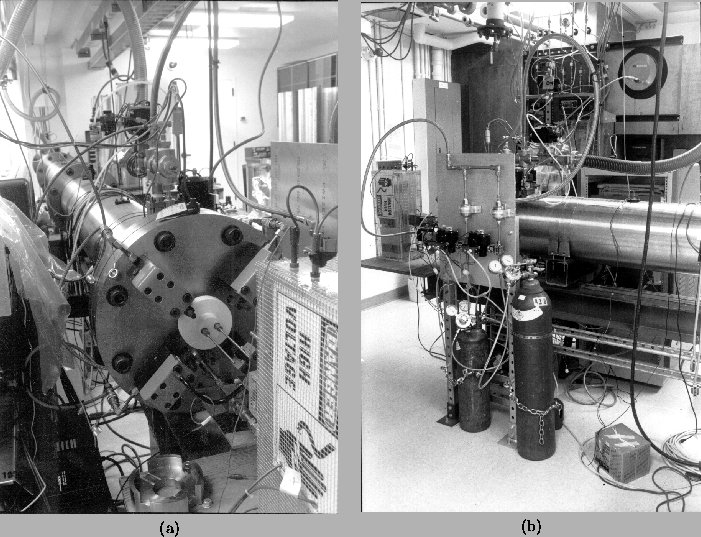 |
Table 1 summarizes the mixtures tested so far.
For all mixtures except mixture 1, air was made from bottled O2and N2. Tests with mixture 1 (H2+N2O+![]() (O2+3.76N2))
used atmospheric air.
We use a simplified representation of air composition as O2 + 3.76N2;
the complete specification of all compositions used in this study
are given in Table 1.
Note that the mixture numbers do not correspond to the mixture numbers
in the previous report [Ross and
Shepherd (1996)].
To simplify the presentation, N2 and air are treated as diluents even
though air is an effective oxidizer. The amount of diluent was specified
in terms of the fraction (percentage) in the figures rather than in terms of the parameter
(O2+3.76N2))
used atmospheric air.
We use a simplified representation of air composition as O2 + 3.76N2;
the complete specification of all compositions used in this study
are given in Table 1.
Note that the mixture numbers do not correspond to the mixture numbers
in the previous report [Ross and
Shepherd (1996)].
To simplify the presentation, N2 and air are treated as diluents even
though air is an effective oxidizer. The amount of diluent was specified
in terms of the fraction (percentage) in the figures rather than in terms of the parameter
![]() given in Table 1 and Appendix
given in Table 1 and Appendix ![]() . For the case
of nitrogen dilution, the fraction of diluent is
. For the case
of nitrogen dilution, the fraction of diluent is ![]() /N where N is the
total number of moles in the mixture formula in Table 1 and in the
case of air, the fraction is 4.76
/N where N is the
total number of moles in the mixture formula in Table 1 and in the
case of air, the fraction is 4.76![]() /N.
/N.
Mixtures 2 to 11 represent simple mixtures of one fuel and one oxidizer that have been used to characterize the behavior of each substance individually. Mixtures 12 through 17 are best estimates of the retained gas composition in the waste tank as determined by recent tests at Hanford. A small percentage of the gas sample was not identified in those cases and was simply stated as ``unknown.'' In those cases, we have increased the amount of N2 to preserve the actual percentages of the other species. For instance, mixture 12 was originally specified with 2% unknown, so the original 33% N2 was replaced with 35% N2. In each series, as the dilution was increased, the initial pressure was increased such that predicted detonation pressures were just below the tube design limit, up to 1 atm initial pressure. The purpose of this strategy was to acquire as much data at 1 atm initial pressure (field conditions) as possible while deviating as little as possible when required by structural limitations. The largest cell sizes possible are about 50% to 100% of the tube diameter (280 mm). Only one test was carried out for each mixture type 3 and 4 and no cell data were obtained.
| Mixture | Composition | Initial Pressure | Note |
| 1 | H2+N2O+ |
100 kPa | |
| 2 | H2+N2O+ |
100 kPa | |
| 3 | 14H2+14N2O+71N2+O2 | 100 kPa | |
| 4 | H2+4O2 | 98 kPa | |
| 5 | CH4+2O2+ |
72-102 kPa | |
| 6 | CH4+4N2O+ |
57-102 kPa | |
| 7 | CH4+4N2O+ |
86-97 kPa | |
| 8 | NH3+0.75O2+ |
66-91 kPa | |
| 9 | NH3+1.5N2O+ |
56-81 kPa | |
| 10 | NH3+1.5N2O+ |
61-101 kPa | |
| 11 | 42H2+21NH3+36N2O+CH4+ |
76-101 kPa | SY-1011 |
| 12 | 29H2+11NH3+24N2O+35N2+CH4+ |
94-101 kPa | SY-101 |
| 13 | 31H2+0.02NH3+4.3N2O+63.08N2+1.6CH4+ |
101 kPa | AW-101 |
| 14 | 63H2+0.02NH3+11N2O+25.28N2+0.7CH4+ |
101 kPa | AN-105 |
| 15 | 47H2+0.02NH3+19N2O+33.08N2+0.9CH4+ |
101 kPa | AN-104 |
| 16 | 61H2+0.05NH3+3.8N2O+35.14N2+0.01CH4+ |
101 kPa | AN-103 |
| 17 | 75H2+2.4NH3+5.6N2O+16.3N2+0.7CH4+ |
101 kPa | A-101 |
Detonation cell widths are measured by the soot foil technique. The cell width is determined by physical measurements of the spacing, transverse to the detonation propagation direction, between triple point tracks inscribed on soot foils placed within the detonation tube. The foils are 61 cm x 91.4 cm x 0.5 mm aluminum sheets, rolled into cylinders to conform to the detonation tube inner diameter. Soot is deposited on the inside surface of each foil by burning a kerosene-soaked cloth strip inside a closed vertical tube containing the foil. Each foil is normally sooted twice, in both vertical orientations, to cancel convection-induced gradients. The upstream edge of the foil is riveted to an aluminum ring (3-mm thick by 51-mm wide) to secure it as the detonation passes. The downstream end (adjacent to the end flange) is clamped at two points to the tube wall. The cell widths are measured on flattened foils, as the transverse distance between triple point tracks. Since this distance can vary significantly over a foil, minimum and maximum values are reported. Note that for small cells (relative to the tube diameter), this is a unique measure of the cell width, but for cell widths on the order of the tube diameter, this measure may not be comparable to measurements in other facilities or by other techniques. In this case, the effect of the tube geometry on the cells should be considered. Currently, cell widths are measured manually. The inherent variation of cell size across the foil and the difficulty of identifying cell boundaries are significant sources of uncertainty and impose serious limitations on efforts to characterize and predict cell size. Typically, 10 cell-width measurements are made and representative minimum and maximum values are reported. In general, the uncertainty in cell-width measurements, reflected in the reported ranges, can be up to 50%.