Our analysis proceeds along dimensional analysis grounds.
The ratio of cell width to reaction zone thickness
(
![]() )
is a function of other nondimensional parameters
of the flow. For a system characterized by a single reaction with
activation energy Ea, energy release q, ratio of specific heats
)
is a function of other nondimensional parameters
of the flow. For a system characterized by a single reaction with
activation energy Ea, energy release q, ratio of specific heats
![]() ,
and detonation Mach number MCJ, we expect that
,
and detonation Mach number MCJ, we expect that
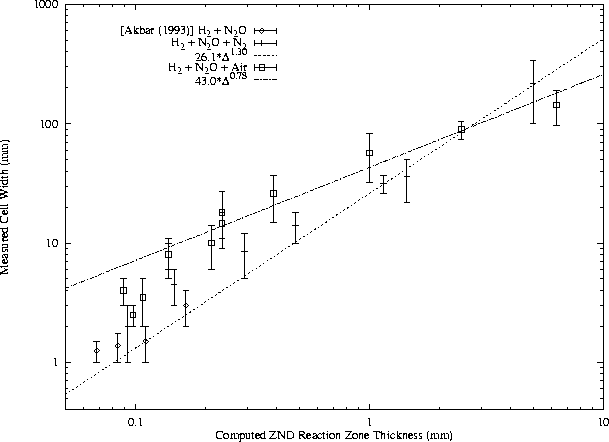
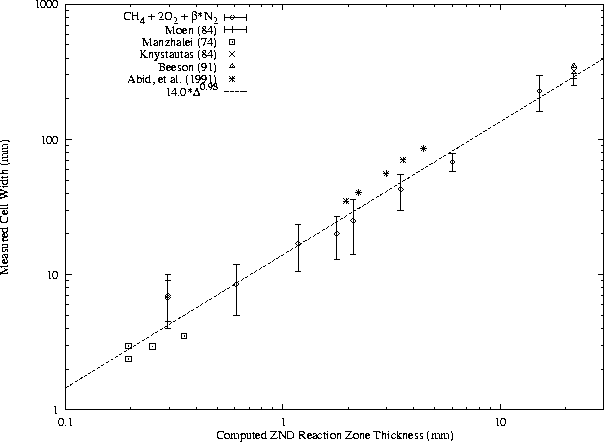
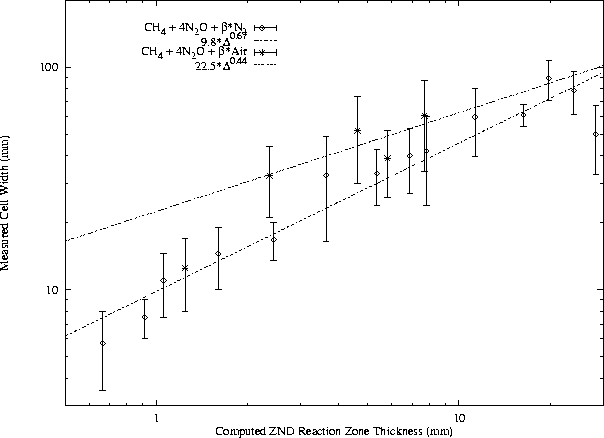
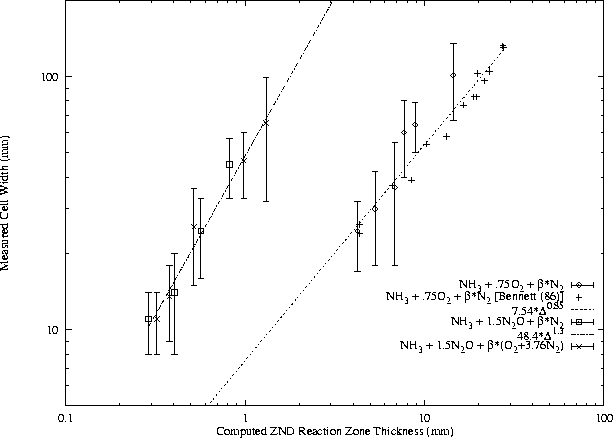
In general, f may include a large number of other parameters, but these are believed to be the most influential. The activation energy and heat release normalization factors include the von Neumann temperature because it is most relevant to the reaction zone behavior. While the general form of this function has not been found, certain useful approximations are possible. For instance, for a given fuel - oxidizer - diluent system at constant equivalence ratio and initial pressure, the function f is generally constant with respect to variation in dilution ratio. A slightly more general approach is illustrated in Figs. 12, 13, 14, and 15. The cell width data from different test conditions are plotted together by using computed reaction zone thickness for each condition as the abscissa.
 |
 |
 |
 |
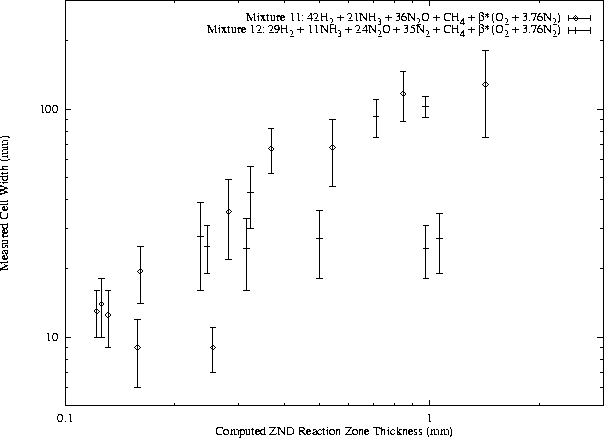 |
For each fuel - oxidizer - diluent system, at constant equivalence ratio, cell width is found to obey a power law with respect to ZND reaction zone thickness. Note that while air is presented as a diluent along with N2 in the H2-N2O and CH4-N2O mixtures, it acts as an oxidizer also and therefore mixtures with air are unique from N2 systems. The undiluted H2-N2O data are considered to be a subset of the N2 dilution data but not the air dilution data because O2 is found to have a significant effect on N2O mixtures even at very small concentrations.
A power law has been least-squares fit to the available cell width - reaction zone thickness data for each mixture, as shown in Figs. 12, 13, and 14. The data for H2-N2O-Air do not correlate well to a power law. The correlations for CH4-O2-N2, CH4-N2O-N2, and CH4-N2O-Air are good, although the number of data points is limited in the N2O mixtures. A number of initial pressures are represented in the literature CH4-O2-N2 data along with various dilution ratios.