



Next: Chemical Reaction Kinetics
Up: Experiments
Previous: Results
Experimental data have been obtained on detonations for mixtures that have
not been previously studied. For convenience, we summarize these data in
Table 2. The diluent amount required to reach a cell width of
100 mm has been estimated by interpolating or extrapolating
the experimental data.
Table 2:
Summary of measured cell width data (initial
pressure of 100 kPa).
| Mixture |
Diluent |
(%) |
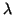 |
| |
|
|
(mm) |
| H2 + 1/2O2 |
- |
0 |
1.3-2.0 |
| |
N2 |
55 |
10-15 |
| H2+N2O |
- |
0 |
1.5 |
| |
N2 |
62 |
36 |
| |
air |
65 |
26 |
| CH4+2O2 |
- |
0 |
3 |
| |
N2 |
72 |
300 |
| CH4+4N2O |
- |
0 |
3(*) |
| |
N2 |
64 |
80 |
| |
air |
60 |
50 |
| NH3+ 3/4O2 |
- |
0 |
16-25 |
| |
N2 |
35 |
100 |
| NH3+ 3/2N2O |
- |
0 |
6(*) |
| |
N2 |
38 |
34(*) |
| |
air |
53 |
64(*) |
| (*) Extrapolated from lower pressure. |
Comparison of results without dilution show that cell widths for H2 and
CH4 are slightly smaller with O2 as the oxidizer than with N2O. The
situation is reversed in NH3, which has a smaller cell width (24 mm vs 40
mm) with N2O as an oxidizer than with O2.
All of the mixtures with the exception of the model SY-101 composition
demonstrate increasing cell widths with increasing diluent concentration.
The SY-101 mixtures show a constant or slightly decreasing cell width with increasing
air concentration up to about 40% dilution, then the cell width rises sharply
with increasing dilution. This is a consequence of
these mixtures being fuel rich.
Air dilution results in slightly smaller cell
widths than N2 dilution for both H2 and CH4 mixtures.
This effect is not discernible in the NH3-N2O data.
The amount of diluent required to obtain a cell width of 100 mm is about 60 to
70% in all cases except for the NH3-O2 mixture which only requires
about 35% N2. The ammonia mixture has substantially
(one order-of-magnitude)
larger cell widths than either the H2 or CH4 mixtures. Using
N2O instead of O2 results in substantially smaller cell widths for
NH3, suggesting a direct channel of reaction that is not present in either
H2 or CH4.
The original SY-101 mixture has a cell width of 100 mm at a dilution of about 75%
air, however the revised composition only requires 60% dilution to reach a
the same cell width.




Next: Chemical Reaction Kinetics
Up: Experiments
Previous: Results
Joe E. Shepherd
2000-01-17